Chronic lung diseases are significant causes of morbidity and mortality worldwide. Asthma, chronic obstructive lung disease (COPD), idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension (PAH), and occupational diseases, including silicosis, affect millions of people around the world.
MSCs are understood as cells that can recognize the microenvironment to they are exposed, modify their phenotype and secretome and, finally, modulate the behavior of other cells. The MSC can induce direct and indirect tissue regeneration, through their anti-inflammatory and immunomodulatory effects on damaged and diseased tissues.
Since MSCs are relatively large cells (average size 30 μm), they interact more with lung endothelial cells. Eighty percent of injected cells are found in the lungs within minutes of IV administration, most of which are cleared in the first week. MSCs are known to have tropism for inflamed or injured sites; thus, when administered in experimental lung disease models, they reach the lungs as a first-line barrier and are attracted by the inflammatory microenvironment.
In chronically diseased lungs, MSCs promote repair through several mechanisms: promotion of reepithelization and neoangiogenesis via secretion of growth factors; reduction of lung edema, in part by decreasing endothelial permeability through induction of synthesis of tight-junction proteins, and in part by increasing epithelial cell reabsorption of fluid through induction of luminal ion channels and basolateral aquaporins; restoration of disease-depleted ATP stores, metabolic capacity, and function of epithelial, endothelial and immune cells, through direct mitochondrial transfer via connexin 43-mediated cell-cell bridges or through extracellular vesicles; protection against microorganisms by secreting antibacterial peptides, and by increasing monocyte phagocytic activity; modulation of inflammation by promoting an anti-inflammatory phenotype in alveolar macrophages (M2 cells) and T regulatory cells, by reducing activity and proliferation of pro-inflammatory cells; and reduction of lung remodeling by reducing collagen fiber content.
Due to the recent COVID-19 outbreak and as there is a lack of medications or vaccines against it, other possibilities are being researched and proven to decrease the intensity of the symptoms and the damage the infection causes to pulmonary tissue. In the more complicated cases, patients suffer from a condition called Acute Respiratory Distress Syndrome or ARDS; this condition is a common cause of respiratory failure, it is characterized by hypoxemia, pulmonary edema, damage to the alveoli and pulmonary “stiffness”. There are multiple medical approaches to the treatment of this condition, but usually, the pulmonary damage is irreversible and causes long term morbidity to the patient. So, what’s one of the new approaches to this therapy? Mesenchymal stem cells.
Stem cells have shown outstanding anti-inflammatory effects by doing immunomodulation, this is done by applying the cells thru an IV, and they have proved to reduce the inflammation by reducing inflammatory mediators of the immune system such as (IL)-1-α, IL-1β, IL-6, IL-8, IFN-γ, macrophage
inflammatory protein (MIP)-1, MIP-2, and tumor necrosis factor (TNF)-α, and by promoting anti-inflammatory mediators such as IL-1 receptor antagonist (IL-1RN), IL-10, prostaglandin
E2 (PGE2), lipoxin A4 (LXA4), and TNF-inducible gene (TSG)-6.
Summary of therapeutic benefits associated with mesenchymal stem cell therapy in experimental acute respiratory distress syndrome[3]
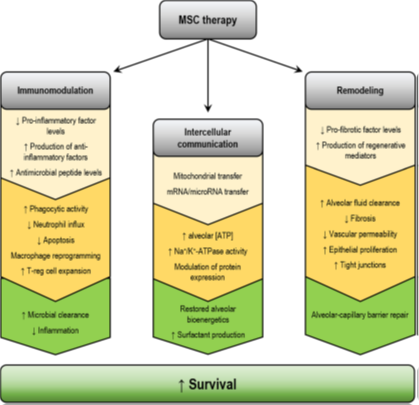
Mesenchymal stem cells can induce the production of TSG-6 factor which is an anti-inflammatory mediator that inhibits the response of innate immune cells, this way they have been able to reduce tissue injury by inhibiting the accumulation of immune cells along the pulmonary tissue and also by inducing the production of IL-1RN to protect lung tissue. When the stem cells are exposed to specific inflammatory cytokines such as TNF-α, they increase de production of prostaglandin E2, which can change the macrophages from an inflammatory polarization (M1 macrophages) to an anti-inflammatory pathway (M2 macrophages). There is also an increased production of IL-10, which can help in the suppression and proliferation of T cells that may cause more extensive damage.
It has also been found that MSCs can reduce apoptosis (programmed cell death) in the lungs and other organs. As infection is the most common cause of ARDS, MSCs have shown to reduce the bacterial load in this case by stimulating phagocytosis by the host immune system.
An essential role of MSCs in the case of ARDS is maintaining the integrity of the alveoli and the capillaries in the lung, which when damaged lead to edema and tissue remodeling (scar tissue), in this case, MSCs preserve and restore the epithelial lining of the alveolar tissue and the endothelium of the vascular system in the lungs reducing the dysfunction caused by ARDS. The in vitro model has shown that MSCs induce protective actions against the disruption of the barrier by modulating vascular endothelial cadherin/β-catenin signaling. In humans, it has also shown a reduction on endothelial permeability on the lungs by inhibiting natural immune cells infiltration of the tissue and increasing the production of fibroblast growth factor (FGF)[14] and also by promoting “adherens” and junction proteins to reduce the amount of inflammatory cells that can bind to endothelium and damage it.
During ARDS the lungs can become filled with fluids, and as we have said there is tissue damage, the clearance of excess of alveolar and interstitial fluid is essential, which causes a decrease on pulmonary surfactant, and by so preventing gas exchange, studies have shown that MSCs improve the clearance by modulating the expression of paracrine factors, channels, and transporters on the cellular membrane. In models of influenza induced lung injury, the stem cells improved the clearance of fluid and protein permeability by enhancing the production of angiotensin I and KGF and by preventing the malfunction of the sodium/potassium ATPase[16].
There are currently several clinical trials on the way, mostly in China, UK, and the United States with excellent results in several cases, but with time being a limiting factor, the long-term outcomes that are yet to be determined. In Sweden, there has been a compassionate use setting in which both patients with severe refractory ARDS who wouldn’t improve under any measures receives systemic administrations of MSCs from a healthy volunteer and both of them recovered from multiple organs, respiratory and hemodynamic failure, both shown severe decrease in biomarkers of inflammation[17].
So, in conclusion, MSCs have shown anti-inflammatory properties, anti-apoptotic effects, enhanced tissue recovery from the blood vessels and the lungs, alveolar clearance of fluid and microbes, improvements in pulmonary injury, and distal organs injury thereby improving the survival of the patient.
Here at bioXcellerator we are hoping to improve the quality of life of our patients and those who surround them, that’s why the whole team is working around the clock to get this kind of treatments cleared and at reach for the population—science by humans for humans.
Karolynn Halpert. MD
Camilo White C. MD
[1] ARDS Definition Task Force et al. 2012
[2] Biehl M, Kashyap R, Ahmed AH, Reriani MK, Ofoma UR,
Wilson GA, et al. Six-month quality-of-life and functional
status of acute respiratory syndrome survivors, compared to
patients at risk: a population-based study. Crit Care. 2015;19:
356.
[3] https://doi.org/10.1007/s10565-019-09493-5
[4] Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor
antagonist mediates the antiinflammatory and antifibrotic
effect of mesenchymal stem cells during lung injury. Proc
Natl Acad Sci U S A. 2007;104(26):11002–7.
[5] Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow
stromal cells attenuate sepsis via prostaglandin E(2)-dependent
reprogramming of host macrophages to increase their
interleukin-10 production. Nat Med. 2009;15(1):42–9.
[6] Chen PM, Liu KJ, Hsu PJ,Wei CF, Bai CH, Ho LJ, et al. Induction
of immunomodulatory monocytes by human mesenchymal
stem cell-derived hepatocyte growth factors through
ERK1/2. J Leukoc Biol. 2014;96(2):295–303.
[7] Pedrazza L, Lunardelli A, Luft C, Cruz CU, de Mesquita FC,
Bitencourt S, et al. Mesenchymal stem cells decrease
splenocytes apoptosis in a sepsis experimental model.
Inflamm Res. 2014;63(9):719–28.
[8] Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C.
Transplantation of menstrual blood-derived mesenchymal
stem cells promotes the repair of LPS-induced acute lung
injury. Int J Mol Sci. 2017;18(4). https://doi.org/10.3390
/ijms18040689.
Silva JD, de Castro LL, Braga CL, et al. Mesenchymal stromal
cells are more effective than their extracellular vesicles at
reducing lung injury regardless of acute respiratory distress
syndrome etiology. Stem Cells Int. 2019a;2019:8262849.
Zhang S, JiangW, Ma L, Liu Y, Zhang X,Wang S. Nrf2 transfection
enhances the efficacy of human amniotic mesenchymal
stem cells to repair lung injury induced by lipopolysaccharide.
J Cell Biochem. 2018;119(2):1627–36.
Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, et al.
Activation of Wnt/β-catenin signaling promotes mesenchymal
stem cells to repair injured alveolar epithelium induced
by lipopolysaccharide in mice. Stem Cell Res Ther. 2015;6:
65.
Devaney J, Horie S, Masterson C, Elliman S, Barry F, O’Brien T,
et al. Human mesenchymal stromal cells decrease the severity
of acute lung injury induced by E. coli in the rat. Thorax.
2015;70(7):625–35.
Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M,
et al. Human mesenchymal stem cells inhibit vascular permeability
by modulating vascular endothelial cadherin/β-
catenin signaling. Stem Cells Dev. 2011a;20(1):89–101.
Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic
effects of human mesenchymal stem cell in ex vivo human
lung injured with live bacteria. Am J Respir Crit Care Med.
2013;187(7):751–60.
Pati S, GerberMH, Menge TD,Wataha KA, Zhao Y, Baumgartner
JA, et al. Bone marrow derived mesenchymal stem cells
inhibit inflammation and preserve vascular endothelial integrity
in the lungs after hemorrhagic shock. PLoS One.
2011b;6(9):e25171.
Chan MC, Kuok DI, Leung CY, et al. Human mesenchymal
stromal cells reduce influenza A H5N1-associated acute lung
injury in vitro and in vivo. Proc Natl Acad Sci U S A.
2016;113(13):3621–6.
Simonson OE, Moudiakakos D, Heldring N, et al. In vivo effects
of mesenchymal stromal cells in two patients with severe
acute respiratory distress syndrome. Stem Cells Transl Med.
2015;4(10):1199–213.