The COVID-19 was first reported on December 31st, 2019, by Chinese authorities to the WHO. (World Health Organization) Since then, there has been a strict follow up of this novel virus by international bodies as it began to be detected in other countries besides China, such as other Asian countries and Europe as well, now found all over the world and declared a pandemic by the WHO[1]. In the beginning, international officials didn’t realize the ability of the virus to spread so quickly; frankly, no one knew much about it. The way it spread and the implications to the human body and its response to the virus, and realizing there were no medications or vaccines available for COVID-19. As of today, there are 826,222 cases throughout the world, with 174,115 recoveries and 40,708 deaths. (Live feed map)
International efforts now are combined in researching effective methods to avoid the spread and manage the infection[2]. Because the researchers all around the world have begun to search for medication, develop vaccines, and try experimental procedures to try to reach a solution that is feasible and safe for the general population.
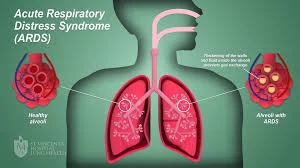
Because of this, researchers in the field of advanced stem cell therapy have come forward to help. They have started trials to assess the efficacy, safety, and outcomes of stem cell therapy in patients with the infection and with Acute Respiratory Distress Syndrome or multi-organ failure due to the immunological body response to the novel infection[3].
MSCs have shown in prior animal studies that, in this case, MSCs or their secreted products (e.g., paracrine factors, exosomes, or mitochondria)[4] can improve oxygenation, and reduce extravascular lung fluid, pulmonary edema, and vascular permeability.[5] [6] [7] [8] [9]. There are also anti-inflammatory and antimicrobial effects observed. MSCs from qualified donors have exhibited an outstanding safety record in a wide range of clinical trials, spanning a large number of patients and delivery modalities. These exogenously delivered cells then have been consistently shown to home toward zones of injury, including the lung, where they can mitigate the progression of damage or potentially even restore or repair cellular functions. The sudden, bilateral damage to the lungs via coronavirus and its specter of permanent damage to the resident stem cells and endogenous regeneration capacity is documented by infectious disease experts[10].
What has been observed in vitro and in vivo studies so far, MSCs could be a treatment for patients with COVID-19. Both the infection and its complications post-infection because of the MSC’s ability to regenerate damaged tissue. Research must and will continue towards finding a cure and solving a problem that involves the whole world, being MSCs a plausible solution and already at the reach of humanity. What remains now is to start doing treatments and evaluate the responses of the studied individuals with the help of governments all around the world—allowing the therapy to be available to whoever needs it and at a proper time frame.
Advanced Cellular and Regenerative Medicine
References:
[1] http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
[2] https://www.ncbi.nlm.nih.gov/pubmed/31986264
[3] https://celltrials.org/cells-data
[4] https://www.ncbi.nlm.nih.gov/pubmed/?term=21726829
[5] https://www.ncbi.nlm.nih.gov/pubmed/?term=31701782
[6] https://www.ncbi.nlm.nih.gov/pubmed/?term=32109144
[7] https://www.ncbi.nlm.nih.gov/pubmed/?term=30455077
[8] https://www.ncbi.nlm.nih.gov/pubmed/?term=31647191
[9] https://www.ncbi.nlm.nih.gov/pubmed/?term=24891325
[10] https://www.roosterbio.com/cell-therapy/scalable-msc-manufacturing-matters-in-a-rapid-response-to-covid-19/