I agree with regulatory oversight, but shouldn’t government agencies like the FTC prove the merits of their claims before publishing their accusations?

When I got this letter from the FTC one morning back in June, I was immediately alarmed:
This “warning letter” from the Federal Trade Commission says that BioXcellerator is “unlawfully advertising” and making “unsubstantiated claims” about the effectiveness of certain “products and services.”
But after I read it, that shock turned into confusion. I was baffled. A “warning letter”? Hardly. It’s more like an “accused, tried, and convicted” letter. The letter doesn’t even begin to explain what the agency thinks we did wrong. It’s bizarre that a letter about unsubstantiated claims doesn’t include any proof or explanation.
And to make matters worse, it’s really just a form letter that merely quotes some of our recent blog posts about various therapies now being studied to alleviate serious symptoms suffered by COVID-19 patients.
Proud to participate in clinical trials
Let me be clear: If the FTC had done any investigation at all, they would have found that BioXcellerator has plenty of competent and reliable scientific evidence to support the content we published.
Not only do we treat patients with stem cell therapies, we’re proud to actively participate in clinical trials. In fact, this research study listed at clinicaltrials.gov, a medical research site managed by the U.S. National Institutes of Health is one of many ongoing studies on treating acute respiratory distress syndrome (ARDS), which is a common and severe complication of the COVID-19 virus.
What’s more, the U.S. Food and Drug Administration (FDA) encourages “expanded access for compassionate use” to help patients learn of potential options and access alternative therapies, such as stem cell therapy to treat severe cases of any disease.
The FTC cites these three posts as “evidence” that we made misleading claims:
A recent study explains how the coronavirus binds to cells and creates a harmful inflammatory response and how “immunomodulation” has been shown to mitigate this inflammation. We cited studies with data that suggests how Wharton jelly mesenchymal stem cells can help modulate the immune system. We made no claims that we could “cure” COVID-19 patients.
We published information on what has been “observed in vitro and in vivo studies so far” on the use of stem cells for treating acute respiratory distress syndrome. There were no claims of any cure. We even concluded the post by suggesting that “research must and will continue towards finding a cure and solving a problem that involves the whole world.” We also pointed out that we need to “start doing treatments and evaluate the responses of the studied individuals…” What’s misleading about that?
https://www.bioxcellerator.com/blog/phenomenal-results-of-iv-stem-cell-therapy-on-chronic-lung-disease , we discussed how ongoing research on stem cells have demonstrated anti-inflammatory effects which can help patients. We even cited an NIH study (among others) on how certain stem cells can reduce death of cells in the lungs. It’s what scientists agree that the data suggests, no claim of a cure.
According to the FTC letter:
“It is unlawful under the FTC Act, 15 U.S.C. § 41 et seq., to advertise that a service or product can prevent, treat, or cure human disease unless you possess competent and reliable scientific evidence, including, when appropriate, well-controlled human clinical studies, substantiating that the claims are true at the time they are made. “
As you can see, we never “advertised” a service or product. And we did indeed cite “well-controlled human clinical studies” that back up the information we did publish.
The FTC’s claims in their letter are not only unsubstantiated – they’re just plain wrong.
A form letter: Long on words. Short on actual content.
What is really annoying that this so-called “warning letter” is a noticeable lack of any actual content: Here’s an overview of how it’s organized:
There’s no substance at all in this warning. It’s merely a form letter.
About 80% of this four-page letter merely quotes our blog posts. Outside of an introduction that tells us we did something wrong and a conclusion that tells us to remove the posts and respond within 48 hours, there’s nothing except cut-and-paste excerpts from our posts. I’ve seen other letters sent to other companies like this and they all seem alike, except for the quoted content.
Shouldn’t we be given the courtesy of at least some interpretation of what the FTC found “unlawful.” I guess in the realm of administrative law, those accused of wrongdoing can’t defend themselves. The result? Instant conviction and sentence imposed.

Not just a warning: Punished in advance.
You might say, “Eric, it’s just a warning, what’s the big deal?”
It is a big deal. For one thing, certain Google searches for our company now include this warning letter near the top of the search results. It seems as if the FTC not only wants to issue “warnings,” it wants to call attention to them by influencing search engine results. The agency spotlights even more attention to them by issuing press releases like these:
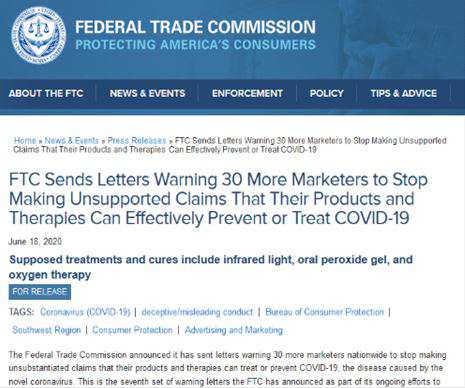
At least I know we’re not being singled out. We were one of 30 companies sent warning letters that same day.
Our reputation has indeed been damaged. Just after the letter was issued, our bank decided to close our merchant account – based on nothing other than this letter – and we had to open another account at another bank.
So yes, it feels almost like we’ve been found guilty of something. That’s unfair and uncalled for.
No opportunity to respond?
Other than this post, do we get the opportunity to explain what we published on our blog and why the FTC is mistaken?
Actually, we not only had the opportunity to respond, we were required to respond within 48 hours.
But while the FTC seems so delighted to issue warning letters and publicize them, does our response get similar attention?
Nope. Take a look. On the FTC’s page about warning letters, it confirms that while their letters are made public, responses “usually are not”:

I agree that there needs to be some governmental oversight of companies that treat patients for serious medical conditions. And I will admit that there are plenty of shady organizations out there making all sorts of product claims that can’t be substantiated at all. But we’re not one of those organizations and never have been.
It’s disappointing that while the government gets a chance to make their warnings public in a way that’s so disrupting (such as requiring us to change banks), I don’t get a fair opportunity to respond.
So yes, I think the FTC is making an “unsubstantiated” claim about the content we published on our blog. With no real recourse, there’s really nothing I can do except get back to work helping others lead better and healthier lives. I think I can make that claim.
I hope fewer CEOs like me have to spend time on issues like these, but it happens.
Don’t say I didn’t warn you!
– Eric Stoffers, CEO/Founder Bioxcellerator