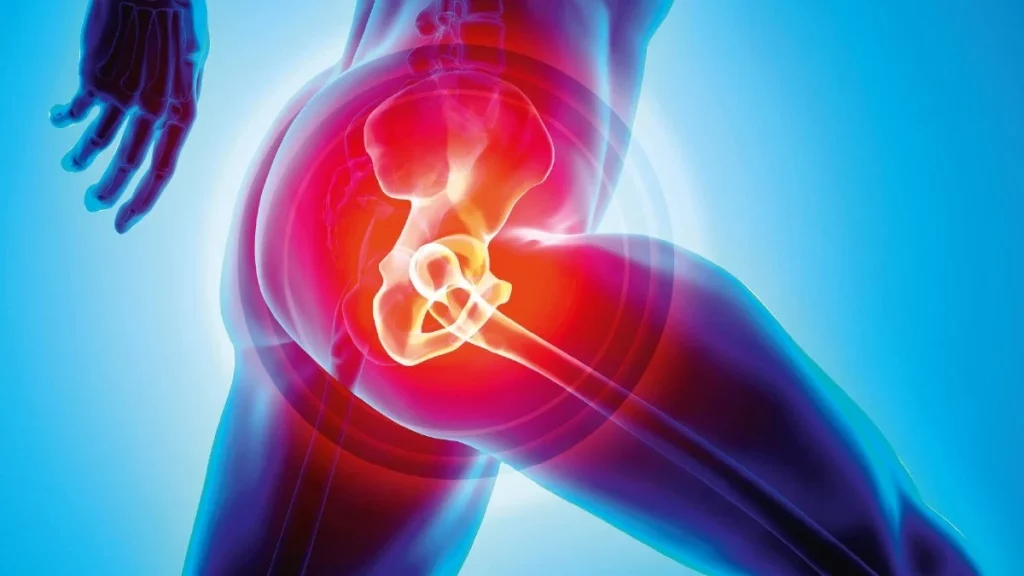
Imagine waking up each morning to the familiar sensation of pain in your lower back, a discomfort that seems to radiate down your legs and hinder your ability to move freely. Every step you take, every time you bend over, and even when you sit down to rest, the pain is always there, like an unwelcome guest that has overstayed its welcome. This is the daily reality for millions of people who suffer from a condition known as degenerative disc disease (DDD).
DDD is a common ailment that affects the discs in the spine, causing them to deteriorate over time. These discs, which are the cushion-like structures between the vertebrae, play a critical role in maintaining the flexibility and stability of the spine. However, when they start to wear down, the consequences can be devastating. The degeneration of these discs can lead to chronic lower back pain, a condition that is often accompanied by radicular symptoms—pain, numbness, tingling, or weakness that radiates along the path of the affected nerves. For those living with DDD, even the simplest tasks, like bending over to tie their shoes, can become a struggle. The condition can take a toll on their mental and emotional well-being, leaving them feeling hopeless and frustrated.
The economic and social impact of DDD cannot be overstated. Lower back pain is one of the leading causes of disability worldwide, and its prevalence has major implications for healthcare systems, employers, and society as a whole. Countless hours of productivity are lost each year as individuals grapple with the challenges of managing their pain. For some, the discomfort becomes so severe that they are unable to work or engage in the activities they once enjoyed. The result is a significant decrease in their overall quality of life.
Despite the widespread prevalence of DDD, effective treatment options are surprisingly limited. Traditional conservative therapies, such as physical therapy, pain medication, and lifestyle modifications, may provide some relief, but they are often insufficient for those with severe or persistent symptoms. Consequently, many individuals feel compelled to turn to surgical interventions for relief. While surgery can be beneficial for certain patients, it is not without its risks. Complications, such as infections, blood clots, and nerve damage, can occur, and there is no guarantee that surgery will ultimately resolve the underlying problem. Furthermore, the invasive nature of surgery and the lengthy recovery process can pose additional challenges for patients.
Amidst this landscape of limited options and uncertain outcomes, researchers have been tirelessly exploring new and innovative approaches to treating DDD. One such approach that has garnered attention in recent years is the use of mesenchymal stem cells (MSCs). These cells, which are naturally found in the body’s bone marrow, possess unique regenerative properties that make them ideal candidates for addressing the underlying causes of disc degeneration.
A study, Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy, was conducted to evaluate the effects of stem cell therapy on patients with DDD.
Results of the Study
The pilot study, conducted by a team of forward-thinking scientists and clinicians, was groundbreaking in its approach to addressing the challenges of DDD. Recognizing the limitations of traditional treatment methods and the need for more effective solutions, the researchers turned their attention to the potential of MSCs as a novel therapeutic option. The decision to explore the use of MSCs was not made lightly; it was informed by years of preclinical research and a deep understanding of the biology of stem cells and their regenerative properties.
The study enrolled a total of 33 patients who were experiencing chronic lower back pain and radicular symptoms as a result of DDD. Each of these individuals had a history of conservative treatment options that had failed to provide adequate relief. For many of them, surgery was the next step, but they were hesitant to undergo invasive procedures with uncertain outcomes. Instead, they chose to participate in the pilot study, which offered the possibility of a less invasive and more innovative solution.
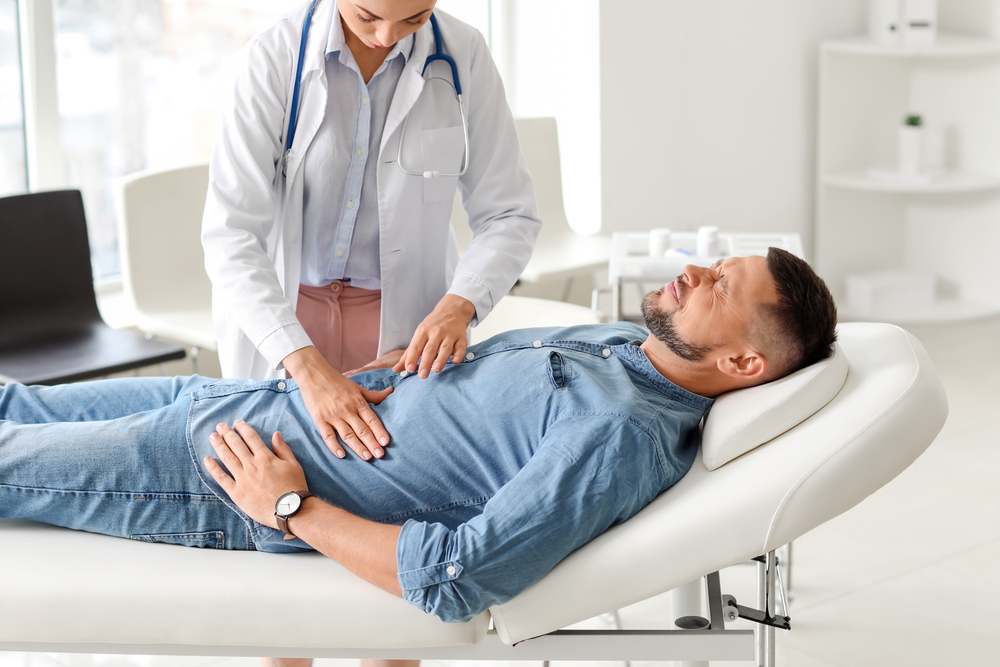
The treatment process began with the careful extraction of bone marrow from each patient’s hip. This bone marrow sample served as the source of the autologous MSCs, meaning the stem cells were derived from the patients’ own bodies. This approach was chosen to minimize the risk of immune rejection and other complications associated with donor-derived cells.
Following extraction, the MSCs were isolated and cultured in a controlled laboratory environment. During this process, the stem cells were expanded to obtain a sufficient number of cells for the treatment. The researchers also took care to ensure the purity and viability of the MSCs, as these factors are critical to the success of the therapy.
Once the MSCs were ready, the patients underwent a percutaneous intradiscal injection—a minimally invasive procedure that involves injecting the stem cells directly into the affected intervertebral discs of the spine. The goal of the injection was to harness the regenerative potential of the MSCs to promote healing and repair within the degenerated discs.
Throughout the study, the researchers closely monitored the safety and efficacy of the treatment. Safety was a primary concern, as the introduction of stem cells into the body comes with inherent risks, such as the potential for uncontrolled cell growth or infection. To the researchers’ relief, the treatment was well-tolerated by the patients, with only three individuals experiencing pain related to the procedure, which resolved over time. Importantly, no serious adverse events, such as infections, tumors, or death, were reported.
The study also yielded promising results in terms of efficacy. Over the course of a six-year follow-up period, the researchers observed significant improvements in the patients’ pain levels, functional abilities, and overall well-being. Notably, a majority of the patients experienced a reduction in the size of their disc bulges, a common feature of DDD that contributes to nerve compression and radicular symptoms.
In addition to these objective measures, the researchers also collected subjective feedback from the patients regarding their experiences with the treatment. Many patients reported substantial improvements in their quality of life, with increased mobility and a newfound ability to engage in activities they had previously avoided due to pain.
The findings from this pilot study, while preliminary, have profound implications for the future of DDD treatment. The use of autologous MSCs as a minimally invasive therapy holds the potential to revolutionize how we approach this common and debilitating condition. By targeting the root cause of disc degeneration and harnessing the body’s own healing capabilities, MSC therapy offers a new path toward lasting relief and improved quality of life for individuals living with DDD.
It is important to recognize that this study represents an early step in the journey toward harnessing the full potential of MSCs for the treatment of DDD. Further research, including larger and more rigorous clinical trials, is needed to validate and refine the approach. Nonetheless, the results of this pilot study are undeniably promising and provide a strong foundation upon which future investigations can build.